Did you know that competent complaint handling systems can noticeably promote business growth?
For instance, nearly 70% of customers who voice complaints are inclined to continue with a company if their concerns are resolved and even 95% if the complaints are mitigated immediately, as per the customer service data collected by ABa Quality Monitoring Ltd.
Product Holds/ Product Release Delays/ Supply Chain Disruptions/ Complaint Resolution = Money on Hold
This is where conducting a comprehensive root cause analysis (RCA) process plays a critical role.

Rather than a one-size-fits-all solution, RCA must comprise a highly adaptable array of tools, methods, and techniques. Anyhow, the following are the steps on how to perform root cause analysis:
Step 1: Comprehend the Problem
Define the problem clearly, ensuring it's specific and measurable.
Step 2: Gather Information
Gather all relevant data, including reports, statements, and documentation.
Step 3: Determine Root Causes
Identify immediate and contributing root causes using RCA techniques.
Step 4: Develop and Employ Corrective and Preventive Actions
Brainstorm and prioritize corrective and preventive actions.
Step 5: Establish an Organized Plan
Establish a detailed plan, allocate resources, and assess risks.
Step 6: Monitor and Verify
Continuously monitor and verify the effectiveness of solutions through training, audits, and process improvements.
5 Whys
The 5 Whys technique is a simple and effective tool for progressively seeking comprehensive answers through a series of probing questions by asking “why” multiple times.
It involves asking “Why did this problem occur?” repeatedly (usually five times) to uncover the root cause behind each answer.
The purpose of the 5 Whys is to steer clear of making assumptions. The responses become increasingly precise. Ideally, the final WHY should reveal a flawed process that can subsequently be rectified.
Fishbone Diagram (Ishikawa or Cause-and-Effect Diagram)
A Fishbone Diagram is a visual representation of potential causes of a problem and derives its name from its resemblance to a fish skeleton.
This diagram features a central line, akin to the backbone of a fish, with spines radiating from both the upper and lower parts of it such that each spine serves as a repository for problem causes. It ultimately converges at the location corresponding to the “head” of the fish, symbolizing the core problem itself.
It helps teams brainstorm and categorize potential causes into categories like people, processes, equipment, environment, and materials until we arrive at the correct one.
FMEA (Failure Modes and Effects Analysis)
FMEA is a structured way of evaluating and attending to the potential failure modes of a product or process and their consequences on the system before an adverse event occurs.
This method proves valuable in the context of root cause analysis by providing a list of likely failure points warranting investigation.
It assigns a risk priority number (RPN) to each potential failure mode based on severity, occurrence, and detectability.
The goal with mitigation or corrective action is to reduce the overall RPN for each identified risk.
DMAIC (Define, Measure, Analyze, Improve, Control)
DMAIC is a data-driven improvement cycle or framework used in Six Sigma that breaks down problem-solving into the following five steps:
Define: Define the problem and project goals.
Measure: Collect data to understand the current process performance.
Analyze: Analyze the data to identify root causes.
Improve: Implement solutions to address the root causes.
Control: Put in place controls to sustain improvements.
Pareto Analysis
Pareto Analysis is based on the Pareto Principle, which is premised on the idea that 80% of a project’s benefit can be achieved by doing 20% of the work - or, conversely, 80% of problems can be traced to 20% of the causes.
It is a powerful quality and decision-making tool to yield the necessary facts needed for setting priorities, and by focusing efforts on the vital few causes, it helps allocate resources efficiently for problem-solving.
FTA (Fault Tree Analysis)
FTA is a deductive analysis method used to determine the root causes of a specific event by working backward from the event.
An FTA uses boolean logic to determine the root causes of an undesirable incident.
It uses graphical representations of fault trees to analyze various events and their relationships.
At the top of the fault tree, the undesirable result is listed. From this event, all potential causes tree down from it.
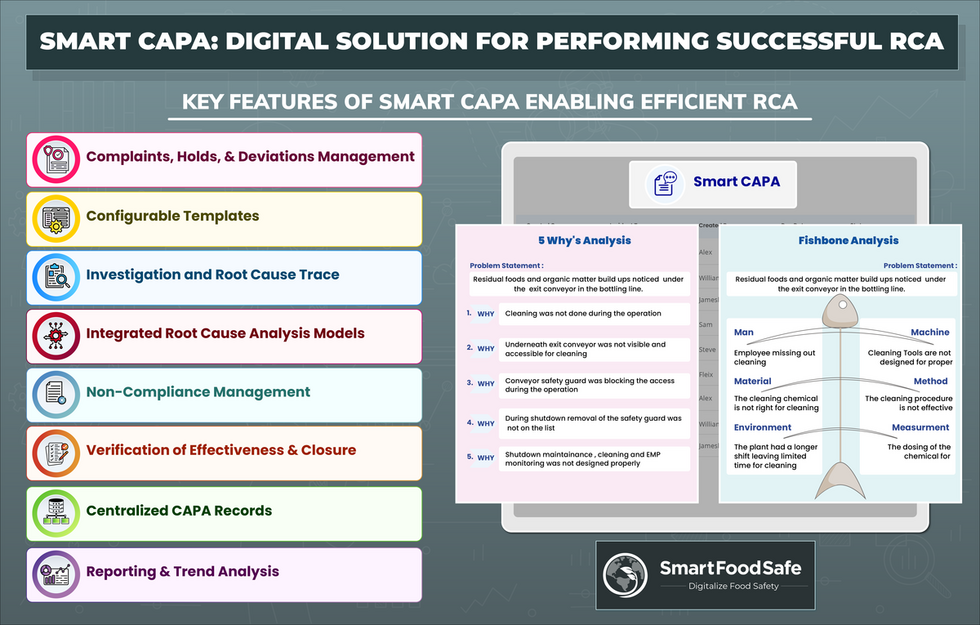
Say goodbye to manual, time-consuming tasks and embrace a streamlined approach to enhance the speed and efficiency of leveraging corrective and preventive actions (CAPA) and root cause analysis processes with Smart CAPA to drive compliance and excellence across your enterprise.
Complaints, Holds, & Deviations Management
Smart CAPA streamlines the entire complaint lifecycle, from investigation to resolution. Digitizing CAPA processes empowers organizations to reduce product holds and deviations, driving continuous improvement.
Configurable Templates
Create customized templates for complaints, deviations, holds, and CAPA, or tailor pre-built templates to match your specific data requirements. Users can effortlessly select the information they need for their records.
Record Creation
Smart CAPA simplifies the generation of comprehensive, auditable records using configured templates. This centralizes the management of extensive CAPA records for easy tracking and retrieval.
Investigation Management
Conduct structured and thorough investigations by digitally gathering relevant data and documentary evidence. This aids in identifying the underlying factors contributing to quality or compliance issues.
Root Cause Analysis
Utilize integrated systematic Root Cause Analysis (RCA) models, such as the 5 Whys or FishBone Analysis, to determine the causes of issues and develop targeted corrective and preventive action plans.
Non-Compliance Management
Plan, define and implement non-compliance action items efficiently. Assign corrective and preventive actions to respective employees for prompt rectification, compliance maintenance, and future risk mitigation.
Verification of Effectiveness & Closure
Evaluate and validate the effectiveness of corrective and preventive actions. Ensure the successful closure of the CAPA process by reviewing and confirming the resolution of nonconformances.
Reporting & Trend Analysis
Detect, correct, and review outliers in your processes, gaining invaluable insights for informed decision-making and continuous improvement.
_1.png)