It was on January 31st, 2023, Food and Drug Administration (FDA) made a groundbreaking announcement proposing the redesign of the Human Foods Program, a decision that came in the wake of crucial insights from an external evaluation conducted by an expert panel from the Reagan-Udall Foundation, as well as an internal review of the FDA’s 2022 infant formula incident.
On 27th June 2023, the FDA unveiled some developments with regard to the Human Foods Program, bringing more clarity to its structure. Dr. Robert M. Califf, Commissioner of Food and Drugs, emphasized that the proposed changes would lead to a revamped framework that is more agile and capable of effectively preventing and responding to emergencies such as recalls.
Over the past few months, a cross-cutting working group of agency officials with expertise in various functional and operational areas has been collaborating to identify opportunities to strengthen operations within the new HFP and ORA. Following this, an update was released, introducing modifications, including moving several of the office’s laboratories and merging its current compliance functions into those of the new HFP and other agency product Centers. These changes are designed to improve coordination, prevention, and response activities across the FDA, ultimately supporting its public health mission more effectively. Given below are the proposed improvements:
ORA’s core mission will be focused on conducting investigations, inspections, and imports for all FDA-regulated products. Assignments will be made in collaboration with the HFP and other product programs or centers. The new Deputy Commissioner for Human Foods will oversee all budget and resource allocations for the entire HFP, including ORA resources.
Compliance functions currently managed within ORA will be merged into the HFP and the compliance functions of other product centers. This streamlining of operations aims to expedite decision-making.
The eight Human and Animal Food laboratories currently managed by ORA will be realigned into the HFP. These labs will join forces with the four labs in the FDA’s Center for Food Safety and Applied Nutrition (CFSAN) to form a unified food laboratory enterprise under the HFP. The labs will report to a member of the executive leadership team under the Deputy Commissioner for Human Foods, who will work closely with the Chief Scientist and Center for Veterinary Medicine (CVM) director to coordinate research priorities. These labs will continue to operate in their current geographic locations.
Certain functions from the Office of Security and Emergency Management, which are currently in the Office of Operations, will be transitioned to ORA. This includes the Office of Emergency Management, which activates Incident Management Groups with augmented staffing from relevant Centers and Offices to coordinate responses to emergency situations involving regulated products, such as recalls, hurricanes, fires, floods, etc.
State and local food safety partnership functions and certain aspects of international food safety partnerships will be unified into an Office of Integrated Food Safety System Partnerships in the HFP. This office will again report to a member of the executive leadership team under the Deputy Commissioner for Human Foods, who will closely collaborate with the CVM director to advance a truly integrated food safety system.
Support functions across ORA will be reviewed, and certain resources and personnel will be realigned to support the proposed changes. This includes staff and resources in ORA’s Office of Regulatory Management Operations, Office of Information Systems Management, Office of Training, and Office of Communications and Project Management.
There will be a focus on recruiting, retaining, and training field-based employees to support the agency’s ongoing efforts to increase inspectional activities domestically and internationally.
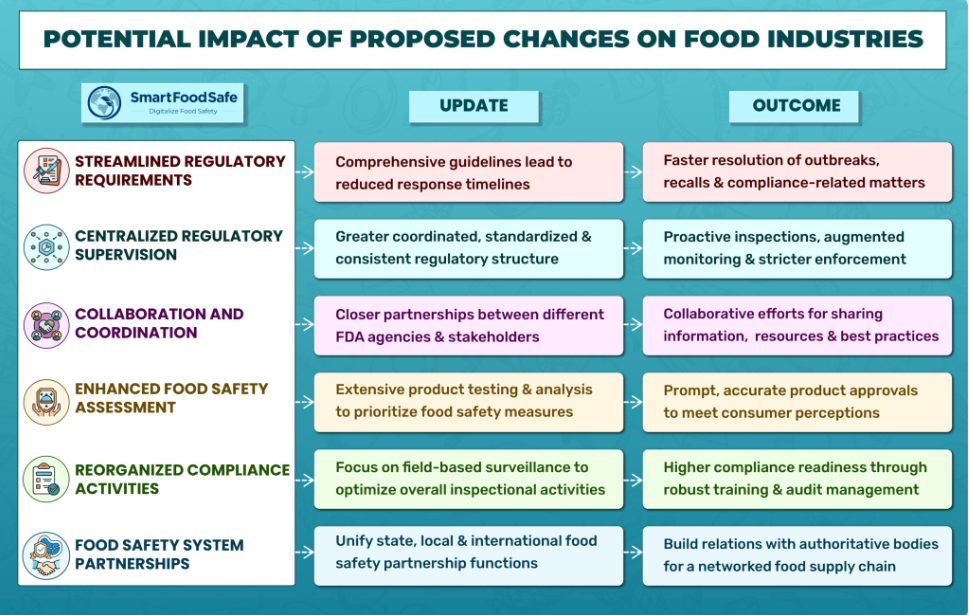
As the FDA undergoes restructuring, the U.S. food industry faces potential impacts on manufacturers and suppliers. The effects will vary based on product type, operation size, and compliance practices. To thrive in this evolving regulatory environment, entities must assess changes carefully, take proactive measures, and collaborate with regulators.
Streamlined Regulations
Consolidation under the Unified Human Foods Program will lead to clearer guidelines and faster responses, easing compliance for food manufacturers and suppliers. Emergency management may improve with efficient communication and response timelines.
Centralized Regulatory Supervision
A more centralized oversight structure could lead to standardized processes and stricter enforcement, requiring adherence to regulations to avoid penalties or recalls.
Collaboration & Coordination
Closer partnerships between the FDA and stakeholders may lead to science-based regulations and influence product development and manufacturing processes.
Reorganized Compliance Activities
Increased field-based inspections will necessitate higher compliance readiness, potentially leading to changes in processes, reporting, and interaction with regulatory offices.
Enhanced Food Safety Assessment
Consumer expectations for food safety and quality may rise, demanding transparency and effective communication from manufacturers and suppliers. The unified food laboratory enterprise could lead to comprehensive product testing and faster approvals.
Food Safety System Partnerships
The Office of Integrated Food Safety System Partnerships may affect how entities interact with regulatory agencies at different levels, encouraging cohesive food safety systems and harmonized regulations across regions and countries.
Smart Food Safe revolutionizes the food manufacturing industry by delivering wide-ranging digital solutions, assisting manufacturers to excel in the evolving regulatory landscape. Our tech-driven suite of digital modules accommodates diverse functionalities, including an integrated centralized data platform, compliance management system, collaboration tools, real-time monitoring, advanced analytics, traceability features, remote auditing capabilities, and predictive risk assessment and quality control. We strive to enable food businesses to master the ability to succeed in the dynamic regulatory environment by prioritizing food safety, building consumer trust, and bringing optimal operational efficiency with ease.
_1.png)