Imagine this; you buy a pack of your favorite sliced mangoes. Would you believe if we say that it is now possible to trace back to their origin farm using blockchain technology in just 2.2 seconds, even before you finish your first delightful bite?
This was a technological advancement brought forth under the revolutionary initiative, the ‘New Era of Smarter Food Safety’ by the U.S. Food and Drug Administration (FDA), championed by the former FDA Deputy Commissioner, Frank Yiannas. This venture aims to leverage new technologies, digital tools and approaches for implementing the science and risk-based requirements of the FDA Food Safety Modernization Act (FSMA) and promote food supply chain traceability and transparency to protect consumers from foodborne illnesses.
As the affordability of such tech-enabled traceability can be a barrier for smaller food enterprises, the FDA New Era of Smarter Food Safety Low- or No-Cost Tech-Enabled Traceability Challenge was introduced on June 1st, 2021. The primary objective of the challenge was to inspire stakeholders, including technology providers, public health advocates, entrepreneurs, and innovators around the world, to develop traceability hardware, software, or data analytics platforms that are accessible at low or no cost for end users, thereby creating traceability systems that are affordable for food businesses of all sizes, encouraging widespread adoption, generating shared value, and facilitating scalability.
Traceability systems serve as the foundation for ensuring food safety, maintaining food quality, substantiating sustainability claims, and setting up transactional mechanisms to mitigate the risks of food fraud and food defense incidents. The much-anticipated Food Traceability Final Rule, published by the FDA on November 21st, 2022, came as a boon for boosting the effectiveness of food traceability systems globally. However, the compliance date of the rule is January 20th, 2026, and there is much to do for the food industry to prepare in these three years due to the complicated nature of the rule.
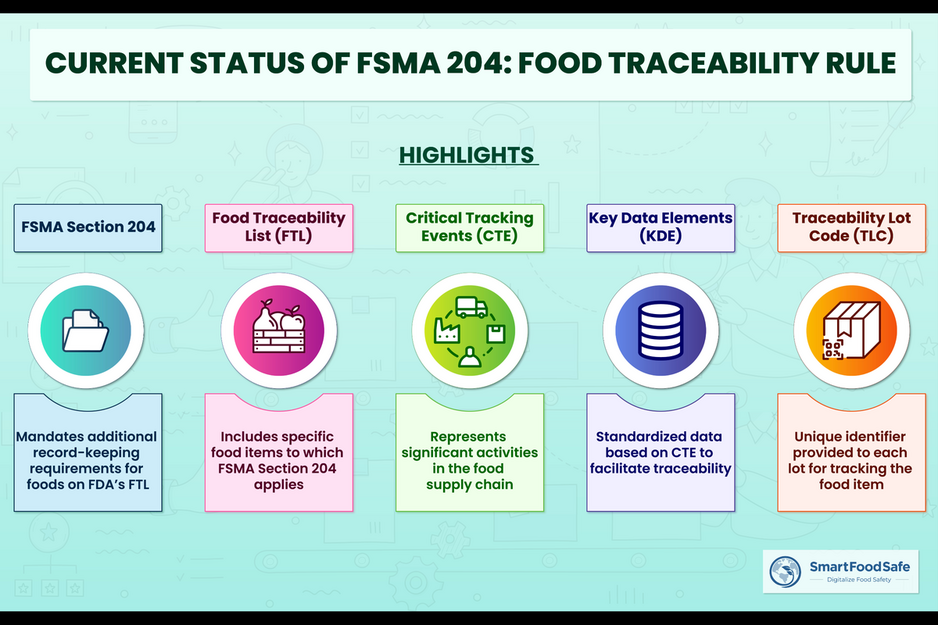
The Food Traceability Final Rule (FSMA Section 204) introduces record-keeping requirements for individuals involved in the production, processing, or packaging of specific foods listed in the Food Traceability List (FTL). The rule applies to FTL foods and foods containing listed items as ingredients, including leafy greens, melons, peppers, and more. Key Data Elements (KDE) based on Critical Tracking Events (CTE) in the supply chain quickly link food safety incidents to specific products. Traceability Lot Codes (TLC) enhance overall traceability, allowing the FDA to track food movement and prevent recalls. Compliance brings a competitive advantage and improved food safety oversight, but challenges related to traceability information, stakeholder processes, and technology must be addressed effectively. Let us have a look at the various challenges faced by the food industry for compliance.
Data Governance and Privacy
To protect privacy, traceability data-sharing procedures require extra attention. Architectural data systems may need modifications for the effective capture of critical tracking events. Inconsistent data formats hinder interconnectivity among supply chain partners, preventing efficient data sharing. Homogeneity, digitization, and unique identification should be prioritized throughout the system. Effective standardization practices are crucial from the start to maintain data reliability and cost-efficiency. Using standards or frameworks can handle supply chain data, ensure clarity on data sources and storage, and identify missing key elements.
Standardized System for Processes
Ensuring consistency and transparency across stakeholders is complex due to variable data formats, incompatible software systems, and technological disparities. Standardized processes are necessary for an integrated traceability system, preventing fragmented records and incomplete data. Weaknesses in current procedures should be identified. Unified data formats, compatible technologies, and common protocols should be implemented for efficient information exchange. This enables rapid response during recalls or contamination incidents, ensuring a reliable and safe food supply chain.
Provision of Globally Unique Identifiers
Lack of adherence to a standardized system hampers traceability. Scannable labels with lot codes should be used for incoming products. Labeling methods like pallet-level or combined-lot labels lack the necessary granularity for traceability. Maintaining a physical-to-digital link using distinct lot codes is crucial. Assessments should identify if lot codes are recorded, traceability gaps in physical processes, and gaps in the physical-to-digital link.
Transfer of Key Data Elements (KDE)
Sharing essential data elements in the supply chain increases compliance risks if upstream partners fail to provide necessary traceability data. Dependency on suppliers for ingredient details may be challenging due to confidentiality concerns. Training is needed to adapt to traceability practices. Agreements among supply chain partners are necessary to exchange traceability data and encourage digitalization. Strategies should ensure compliance, manage exempt suppliers, and establish efficient data transmission to customers.
Management of Traceability Data
Validating and recording traceability data across the supply chain can be complex, especially with outdated systems. Centralizing scattered information is important, especially for timely FDA reporting. Data-sharing systems must accommodate stakeholders with varying technological expertise. Digital records are crucial for managing the large volume of traceability data. Assessments are needed to adjust or improve supply chain technologies, handle growing data volume, and decide whether to manage traceability data internally or through third-party services.
Inspecting the challenges faced by food enterprises to comply with FSMA section 204 by 2026, it becomes evident that it calls for the adoption of new solutions, particularly those driven by automation technology. Thus, what initially appears as a regulatory requirement reveals itself as a chance to revolutionize the industry and achieve higher levels of food safety and quality assurance.
Smart Food Safe’s manifold of software solutions offer end-to-end traceability for modern food production chains and equip the food sector to tackle barriers in achieving compliance with Food Traceability Rule. By deploying automation and digitalization as tools, SmartFoodSafe not only enhances traceability capacities but also complements performance, integrity, and responsiveness throughout the food supply chain, promoting effective recall management and proactive quality assurance measures.
_1.png)